This page/content is for Northern Ireland healthcare professionals only
Pluvicto®▼(lutetium [177Lu] vipivotide tetraxetan), in combination with androgen deprivation therapy with or without androgen receptor (AR) pathway inhibition, is indicated for the treatment of adult patients with progressive prostate-specific membrane antigen (PSMA)-positive metastatic castration-resistant prostate cancer (mCRPC) who have been treated with AR pathway inhibition and taxane-based chemotherapy.1
See the difference with Pluvicto + BSoc*
![]()
Significantly extends overall survival vs BSoC alone2
![]()
Maintains quality of life for longer vs BSoC alone3
![]()
Acceptable safety profile2–4
The recommended treatment regimen of Pluvicto is 7400 MBq intravenously every 6 weeks (±1 week) for up to a total of 6 doses, unless there is disease progression or unacceptable toxicity.1
Medical castration with a gonadotropin-releasing hormone analogue should be continued during treatment in patients not surgically castrated.1
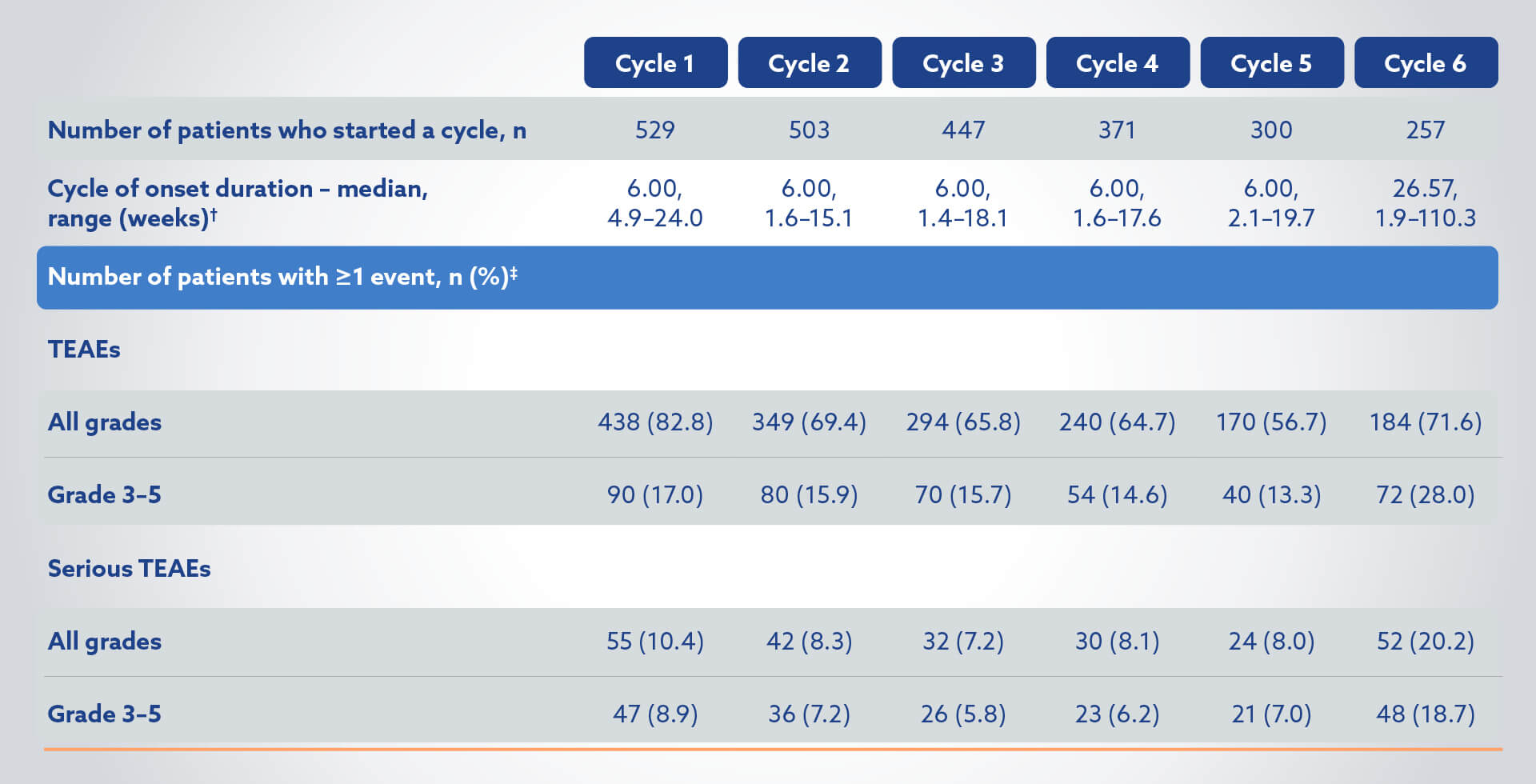
>50% (289/529) received the 5th or 6th cycle of Pluvicto4
TEAEs in the Pluvicto + BSoC group (N=529) by cycle of onset4
Treatment exposure: BSoC exposure was longer in the Pluvicto + BSoC arm vs BSoC alone2
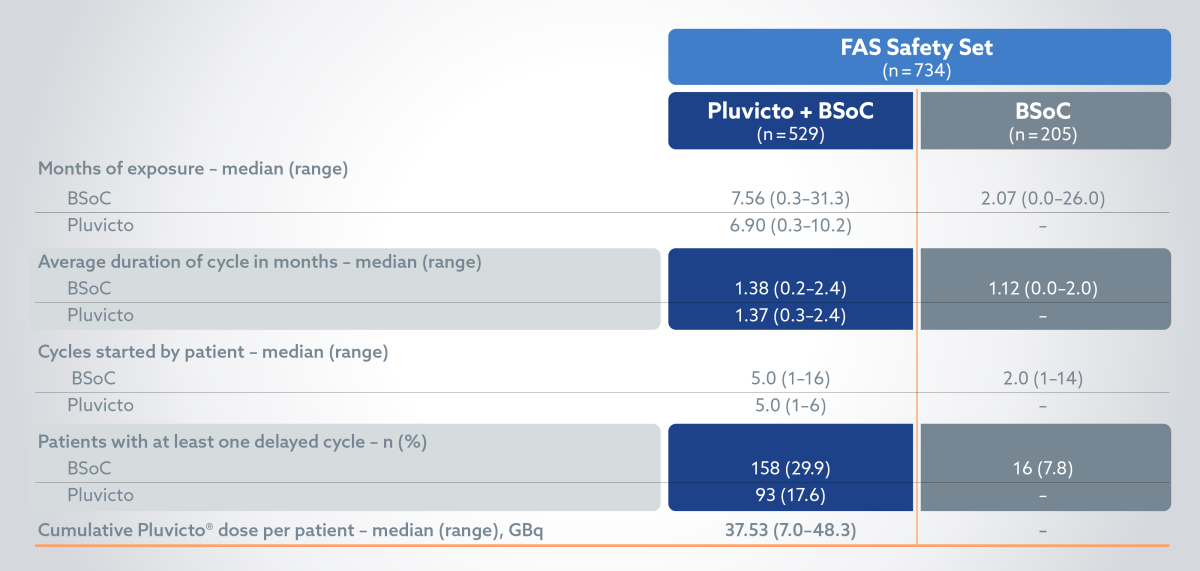
Pluvicto treatment discontinuation due to TEAEs grade ≥32
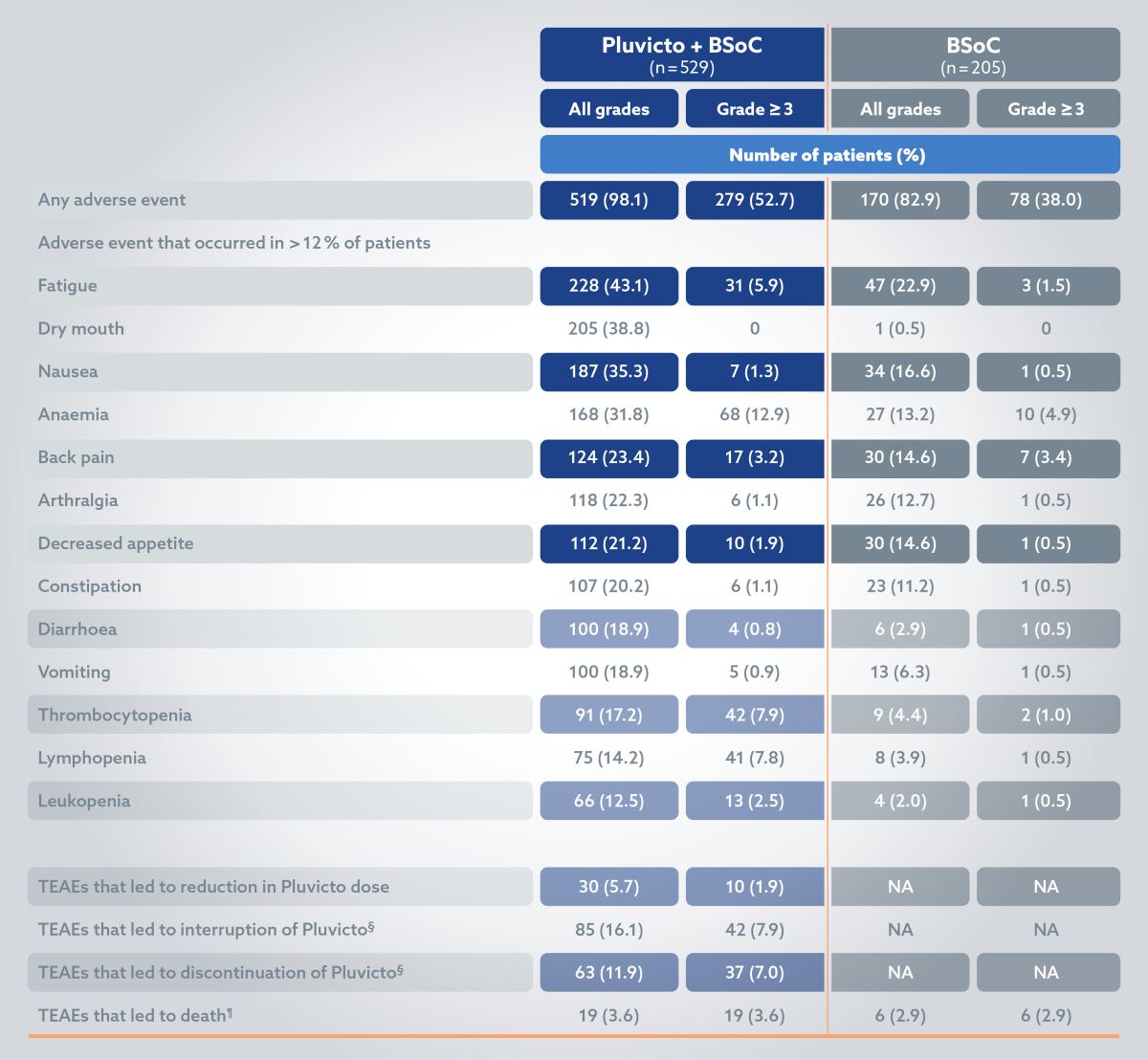
Adverse events2
A range of therapies were permitted for BSoC used in combination with Pluvicto in the VISION trial. Please note Pluvicto is licensed in combination with androgren deprivation therapy with or without AR pathway inhibition only.
The most common ADRs (≥20%) occurring at a higher incidence in patients who received Pluvicto + BSoC compared to BSoC alone include: fatigue (43.1%), dry mouth (39.3%), nausea (35.3%), anaemia (31.8%), decreased appetite (21.2%) and constipation (20.2%).2
Please refer to the Pluvicto SmPC for further information.
*In VISION, a randomised, open-label, multicentre, Phase III clinical trial (n=831), Pluvicto prolonged median overall survival, median radiographic progression-free survival and maintained quality of life for longer when used alongside BSoC compared to BSoC alone. Primary endpoint: radiographic progression-free survival was improved by 5.3 months vs BSoC (8.7 vs 3.4 months, respectively; HR 0.40; 99.2% CI, 0.29–0.57; p<0.001). Alternate primary endpoint: Median overall survival was improved by 4 months vs BSoC (15.3 vs 11.3 months, respectively; HR 0.62, 95% CI, 0.52–0.74; p<0.001). Secondary endpoints: Quality of life measured as FACT-P total score was maintained by 3.5 months more vs BSoC (5.7 vs 2.2 months, respectively; HR 0.54, 95% CI, 0.45–0.66), quality of life measured as BPI-SF pain intensity was maintained by 3.7 months more vs BSoC (5.9 vs 2.2 months, respectively; HR 0.52, 95% CI, 0.43–0.63).2,3
†Cycle duration is time from cycle start to next cycle start (or earliest date of subsequent anti-cancer treatment, and date of last administration of randomised treatment + 30 days). Cycle 6 duration was until the earliest date of subsequent treatment, and date of last administration of randomised treatment (including BSoC) + 30 days.4
‡Onset date on or after current cycle but before start of next cycle. Percentages are based on the number of patients who started the cycle.4
§Patients who had been randomly assigned to receive Pluvicto plus BSoC and who did not receive Pluvicto but did receive BSoC were included in the control group (BSoC alone) of the safety population; 3 patients had adverse events during cycle 1 of Pluvicto therapy that led to the interruption (in 2 of 205 patients [1.0%]) or discontinuation (in 1 [0.5%]) of that therapy.2
¶Five adverse events that led to death in the Pluvicto group were considered by the investigators to be related to the drug: pancytopenia (in 2 patients), bone marrow failure (in 1), subdural haematoma (in 1) and intracranial haemorrhage (in 1).2
177Lu, lutetium-177; ADR, adverse drug reaction; AR, androgen receptor; BPI-SF, brief pain inventory (short form); BSoC, best standard of care; CI, confidence interval; FACT-P, functional assessment of cancer therapy - prostate; GBq, gigabecquerel; HR, hazard ratio; mCRPC, metastatic castration-resistant prostate cancer; PSMA, prostate-specific membrane antigen; TEAE, treatment-emergent adverse event.
References:
- Pluvicto® Northern Ireland Summary of Product Characteristics. Available at: https://www.ema.europa.eu/en/medicines/human/EPAR/pluvicto [Accessed October 2024].
- Sartor O, et al. N Engl J Med 2021;385(12):1091–1103.
- Sartor O, et al. N Engl J Med 2021;385(12):1091–1103. Supplementary Appendix.
- Tagawa ST, et al. J Clin Oncol 2022;(Suppl 16):5047–5047.

