Pluvicto®▼ (lutetium [177Lu] vipivotide tetraxetan) is indicated for the treatment of adult patients with prostate-specific membrane antigen (PSMA)-positive metastatic castration-resistant prostate cancer (mCRPC) who have been treated with androgen receptor (AR) pathway inhibition and taxane-based chemotherapy or who are not medically suitable for taxanes.1
Locametz®▼ (gozetotide) is for diagnostic use only. Locametz, after radiolabelling with gallium-68, is a radioactive diagnostic agent indicated for the identification of prostate-specific membrane antigen (PSMA)-positive lesions by positron emission tomography (PET) in adult patients with prostate cancer.2
Mechanism of Action (MoA)
Pluvicto is a PSMA-targeted radioligand therapy (RLT) that delivers DNA-damaging radiation to PSMA-positive bone, nodal, and visceral metastases.1,3–7
Pluvicto targets PSMA-positive cells, including prostate cancer cells1
The MOA of Pluvicto can be broken down into a few key steps:
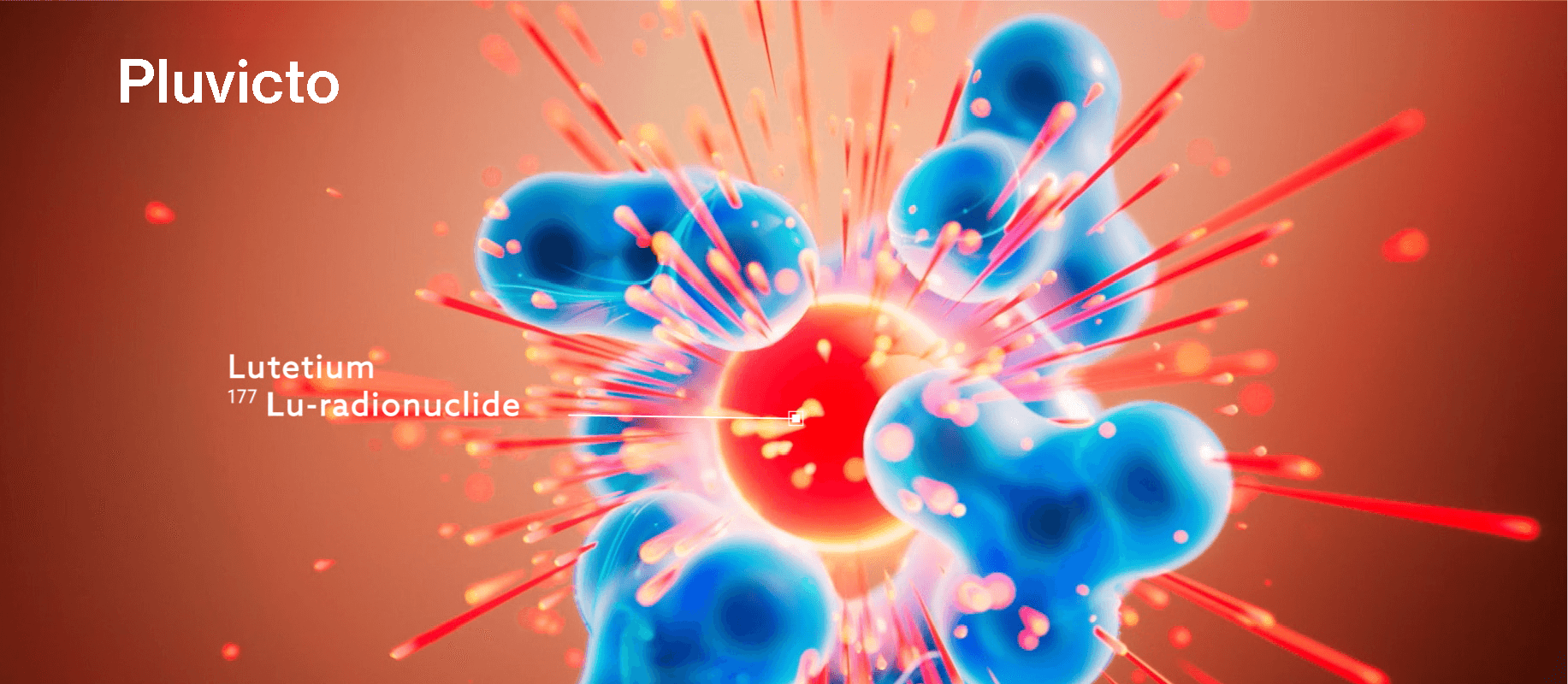
lutetium-177, a cytotoxic radionuclide, and PSMA-617, a PSMA-targeting ligand.1
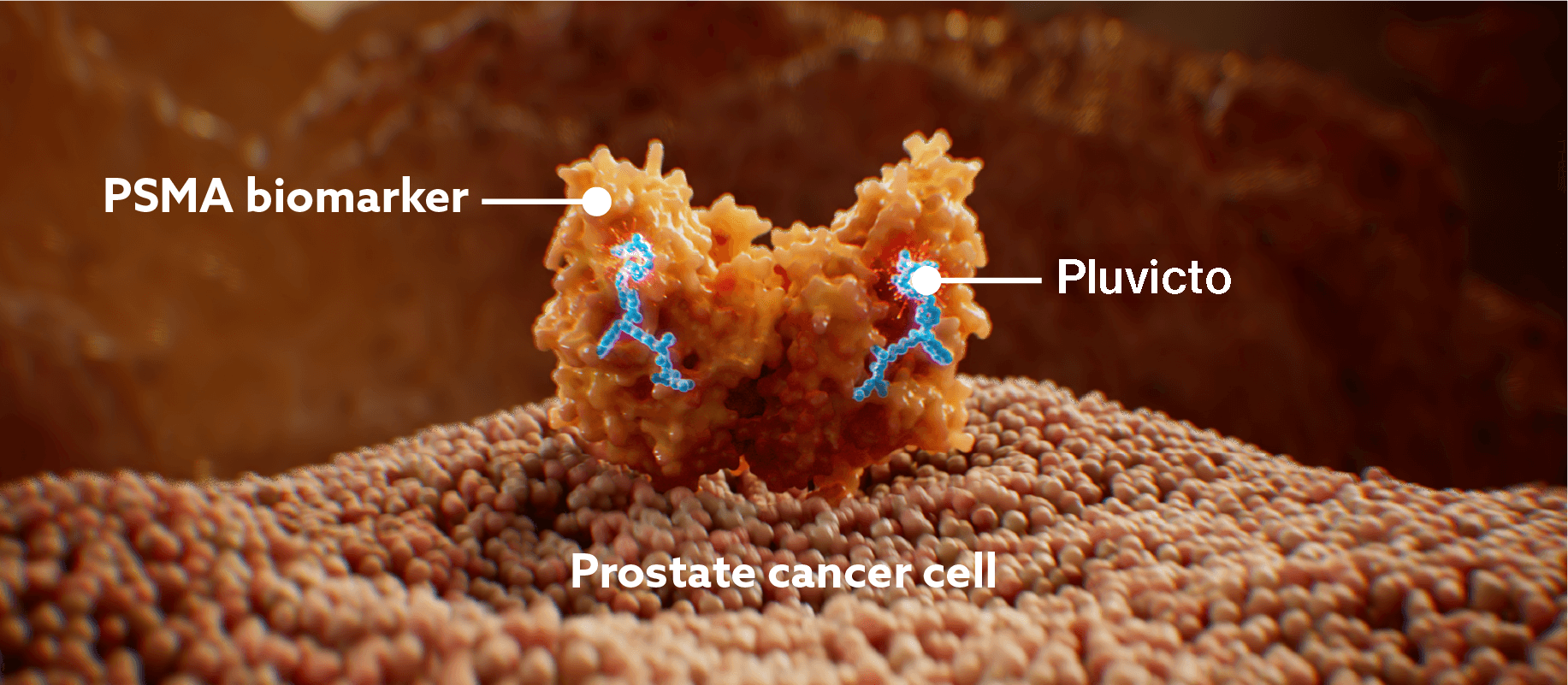
After binding to PSMA, PLUVICTO undergoes endocytosis and is internalised into the cell.1,8–10
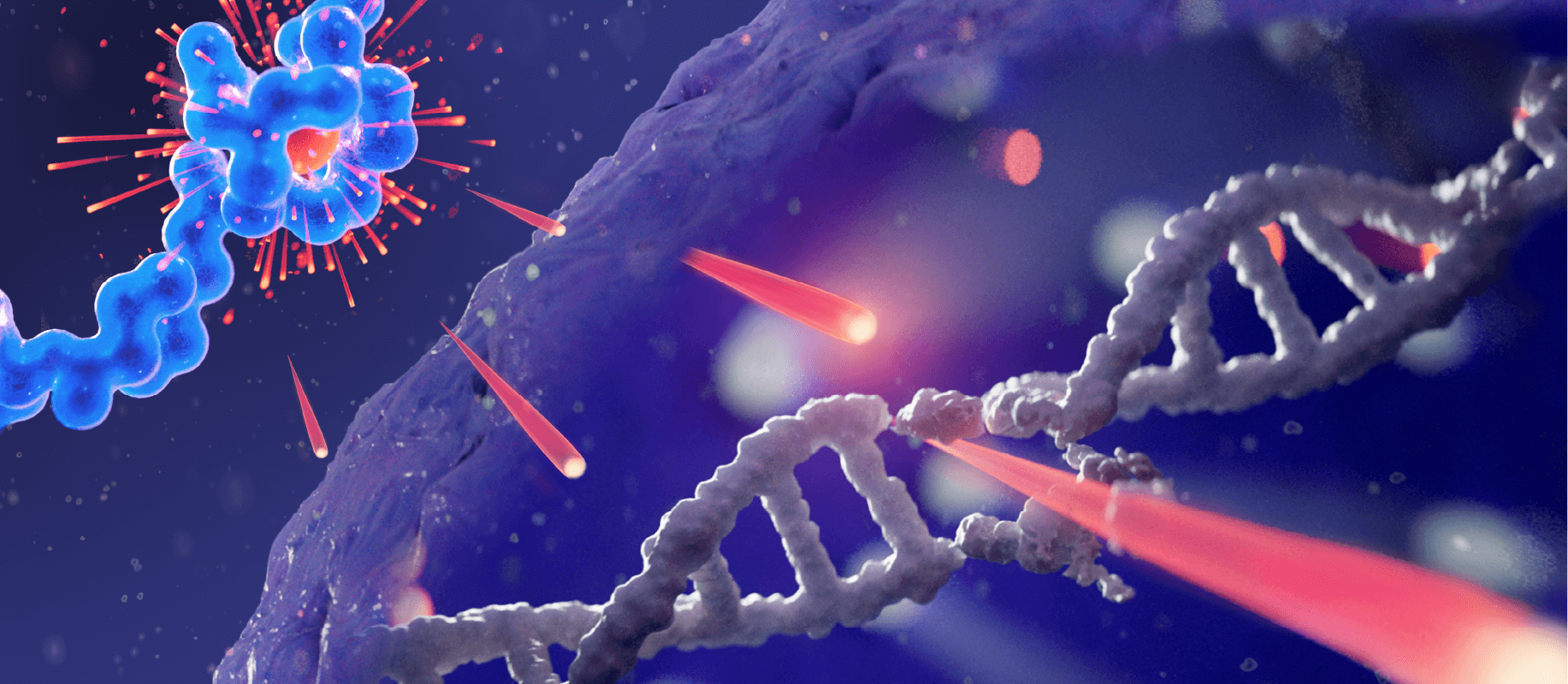
The short path length of the radiation emitted by Pluvicto, (approx. 2 mm max.), causes single- and double-stranded DNA breaks in targeted cells as well as surrounding cells, which can lead to cell death.1,9–11
- PSMA scanning enables you to see and target PSMA-positive mCRPC2,5,13,14
- The high sensitivity of PSMA scanning detects PSMA-positive bone, nodal or visceral metastases13–16
- PSMA imaging establishes patient eligibility for PSMA-targeted RLT6
The most common adverse reactions (≥20%) occurring at a higher incidence in patients who received Pluvicto + BSoC were fatigue (43.1%), dry mouth (39.3%), nausea (35.3%), anaemia (31.8%), decreased appetite (21.2%) and constipation (20.2%).1 Clinically relevant adverse reactions in <5% of patients included dry eye, vertigo and pancytopenia (including bicytopenia).1
Please refer to the Pluvicto Summary of Product Characteristics for the full list of Adverse Reactions.
ADR, adverse drug reaction; AR, androgen receptor; BSoC, best standard of care; DNA, deoxyribonucleic acid; mCRPC, metastatic castration-resistant prostate cancer; MOA, mode of action; PET, positron emission tomography; PSMA, prostate-specific membrane antigen; RLT, radioligand therapy.
References:
- Pluvicto®▼ (lutetium [177Lu] vipivotide tetraxetan) Summary of Product Characteristics.
- Locametz®▼ (gozetotide) Summary of Product Characteristics.
- Benešová M, et al. J Nucl Med 2015;56(6):914–920.
- Fendler WP, et al. J Nucl Med 2017;58(11):1786–1792.
- Hofman MS, et al. Lancet Oncol 2018;19(6):825–833.
- Sartor O, et al. N Engl J Med 2021;385(12):1091–1103.
- Violet J, et al. J Nucl Med 2019;60(4):517–523.
- Liu H, et al. Cancer Res 1998;58(18):4055–4060.
- Rajasekaran SA, et al. Mol Biol Cell 2003;14(12):4835–4845.
- Ruigrok EAM, et al. Eur J Nucl Med Mol Imaging 2021;48(5):1339–1350.
- Sartor O, et al. N Eng J Med 2021;385(12):1091–1103. Supplementary appendix.
- Kassis AI, et al. Semin Nucl Med 2008;38(5):358–366.
- Maffey-Steffan J, et al. Eur J Nucl Med Mol Imaging 2020;47(3):695–712.
- Vlachostergios PJ, et al. Front Oncol 2021;11:630589.
- Woythal N, et al. J Nucl Med 2018;59(2):238–243.
- Schmuck S, et al. J Nucl Med 2017;58(12):1962–1968.

